Articles Menu
A flow battery is an electrical storage device that is a cross between a conventional battery and a fuel cell. (See BU-210: How does the Fuel Cell Work?) Liquid electrolyte of metallic salts is pumped through a core that consists of a positive and negative electrode, separated by a membrane. The ion exchange that occurs between the cathode and anode generates electricity.
Most commercial flow batteries use acid sulfur with vanadium salt as electrolyte; the electrodes are made of graphite bipolar plates. Vanadium is one of few available active materials that keeps corrosion under control. Flow batteries have been tried that contain precious metal, such as platinum, which is also used in fuels cells. Research is continuing to find materials that are low cost and readily available.
Activated by pumps, flow batteries perform best at a size above 20kWh. They are said to deliver more than 10,000 full cycles and are good for about 20 years. Each cell produces 1.15–1.55 volts; they are connected in series to achieve the desired voltage levels. The battery has a specific energy of about 40Wh/kg, which resembles lead acid. Similar to the fuel cell, the power density and ramp-up speed is moderate. This makes the battery best suited for bulk energy storage; less for electric powertrains and load leveling that requires quick action.
The electrolyte is stored in tanks. To increase the energy density, the tank sizes can be doubled using ready-made storage tanks at an estimated cost increase of only 50 percent compared to a new system. When replacing the battery, the electrolyte can be reused, further saving cost. Problem areas are the membranes that tend to corrode and are expensive; additives are said to solve this issue. Figure 1 illustrates the flow battery concept.
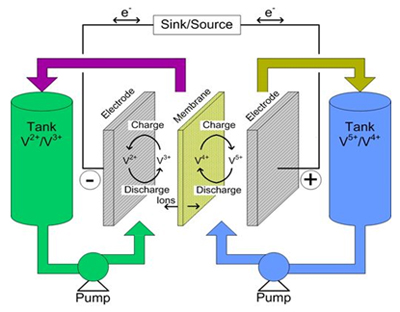 |
Figure 1: Flow Battery. Electrolyte is stored in tanks and pumped through the core to generate electricity; charging is the process in reverse. The volume of electrolyte governs battery capacity. |
Vanadium is the 23rd element on the periodic table and is mined in China, Russia and South Africa. Sun-backed central Nevada may soon become a contributor in the form of heavily oxidized crumbled rock. Currently, 90 percent of lower grade vanadium is used as an additive to strengthen steel. Battery scientists, mining companies and politicians are excited about vanadium becoming a strategic metal for “green energy.” As of now, the cost of the flow battery is similar to that of lithium-ion at about $500/kWh; a future cost of US$250/kWh seems feasible.
For a more precise cost estimation, the flow battery is divided into power cost and energy cost. The power cost can go above $1,500/kW and consists of stacks, pumps, pipes and power electronics. The energy cost consisting of tanks and electrolyte comes in at a bit more than $300/kWh.
Large scale flow batteries exceeding 100kWh have been in use in Japan since 1996. Some of the biggest current installations boast a capability of several megawatts and a flow battery for frequency regulation is being installed in Japan that will deliver a whooping 60MWh.
There is a move towards cost and size reduction. Rather than building a monster battery resembling a chemical plant, newer systems come in container-sizes of typically 250kWh that can be stacked. Modern flow batteries are also becoming common in Europe.
The first patent for a titanium chloride flow battery was granted in July 1954. The present day vanadium redox battery was patented in 1986 by the University of New South Wales in Australia. The term “redox” comes from “electron transfer” of reduction and oxidation.
Last updated 2016-06-15